The ePRO and eCOA Solution That Adapts to Your Study
Clinical trials depend on accurate, timely patient data, yet most ePRO and eCOA solutions make participation harder, not easier. Complex workflows, rigid logic, and outdated UX increase site burden and lead to missing or poor-quality data.
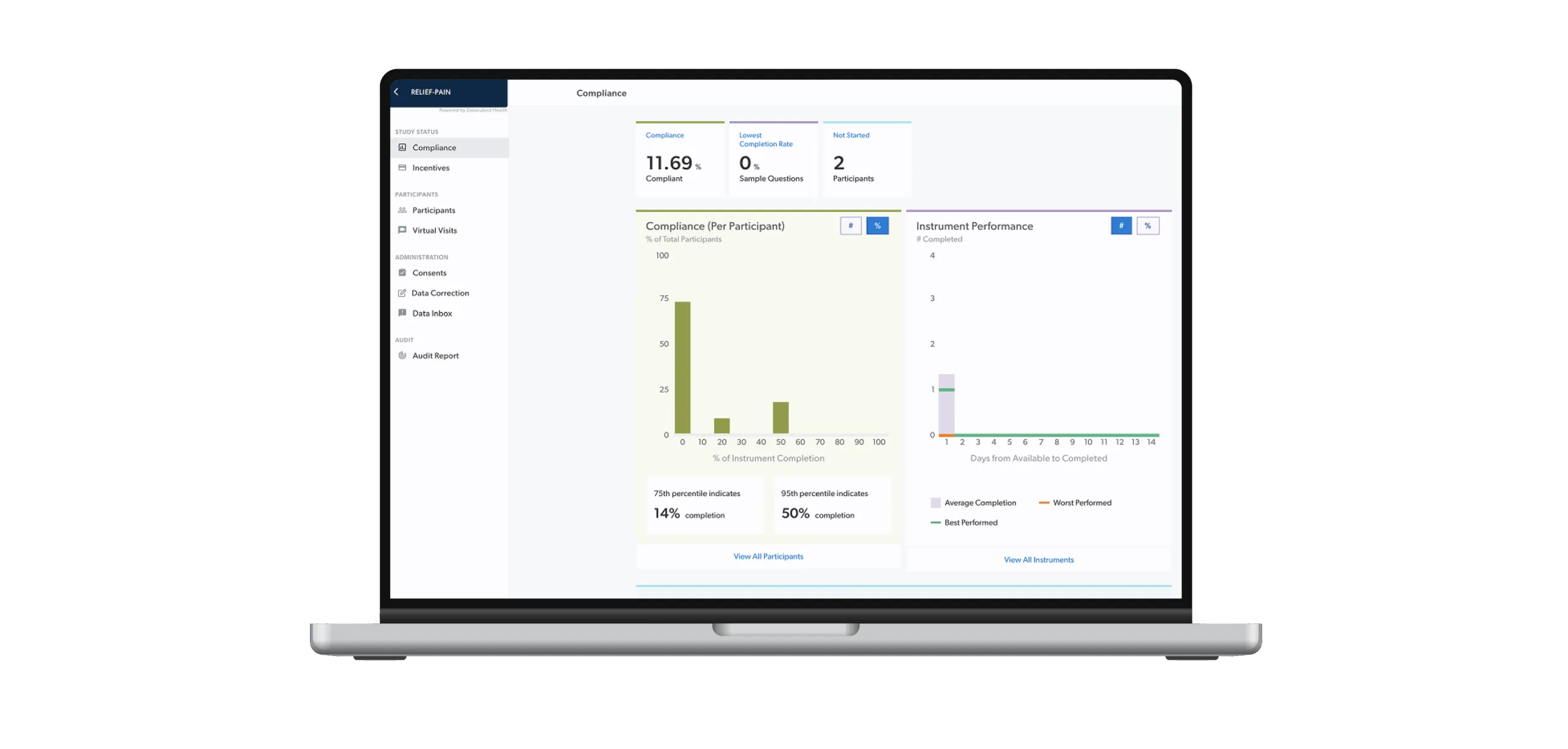
Datacubed takes a different approach. Our clinical research technology is built around human behavior, adaptive design, and real-world workflows. From the simplest diary to the most complex cycle-based oncology study, our platform adapts instantly with no custom code, no long timelines, and no friction, from kickoff to close out.
We bring ePRO and eCOA together into one adaptive environment, allowing participants, sites, and sponsors to interact with the study in ways that feel natural, consistent, and reliable.
Empowering ePRO Solutions That Drive Better Data
Patients stay engaged when technology feels familiar, supportive, and effortless. Datacubed’s ePRO delivers exactly that: a mobile-first experience that meets participants where they are and motivates them throughout their journey.
We designed every detail to build one thing: trust. Here’s how:
- Mobile-first design that feels instantly familiar
- Built-in engagement engine proven to drive 85%+ retention
- Real-time reminders and progress cues that feel supportive, not intrusive
- Optional gamification to boost engagement through behavioral science
- Flexible support BYOD or provisioned devices
- Complex survey logic supporting oncology, CNS, and cycle-based trials
- Real-time data checks for cleaner, higher-integrity datasets
- 90%+ compliance demonstrated across studies
Easy-to-Use eCOA That Simplifies Workflows
Site burden is one of the biggest silent risks in eCOA. When workflows are slow, rigid, or confusing, deviations rise and data quality suffer. Datacubed’s eCOA is built to eliminate that friction, making every step faster, clearer, and more reliable for site staff.

We engineered every workflow to eliminate one thing: doubt. Here’s how:
- Full support for PRO, ClinRO, ObsRO, and PerfO
- Validated, compliant data capture from the first entry
- 21 CFR Part 11, HIPAA, and GDPR compliance
- Audit-ready dashboards tailored to each stakeholder
- Automated workflows that reduce clicks and prevent errors
- Effortless integration with your EDC and site systems
- Centralized oversight across all sites and patients
See the Difference in Real Trials
Explore case studies that demonstrate how our ePRO and eCOA solutions have driven increased engagement, improved compliance, and a better experience.
Experience the Difference
See how Datacubed adapts to your protocol, simplifies workflows, and delivers data you can trust.



