eConsent Software That Builds Trust Early
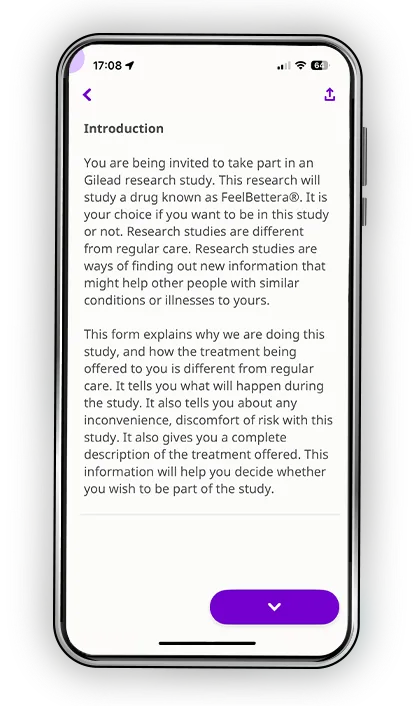
Informed consent is one of the most defining moments in a clinical trial. It’s the moment when a person tries to make sense of complex medical information while deciding whether to take a step that could change their life. Patients deserve more than dense PDFs, rushed explanations, and paperwork that adds stress to an already challenging decision. Datacubed transforms consent into an experience that feels clear, human, and empowering, while giving sites and sponsors the consistency, speed, and compliance they depend on. This isn’t just paperwork. It’s the first chapter of trust.
Comprehensive, Integrated eConsent
Unlike stand-alone tools that fragment workflows, Datacubed’s eConsent lives inside the same ecosystem as ePRO, eCOA, and patient engagement solutions, keeping every part of the participant journey in one place.
For Patients: Consent That Meets People Where They Are
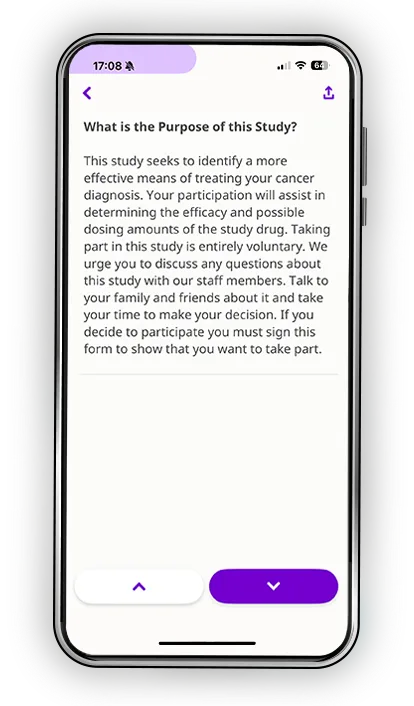
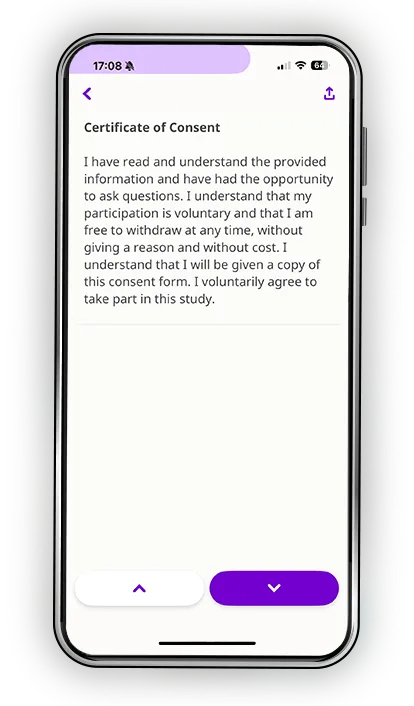
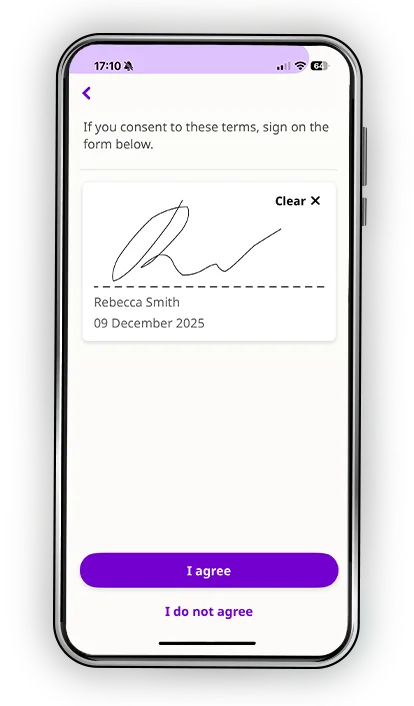
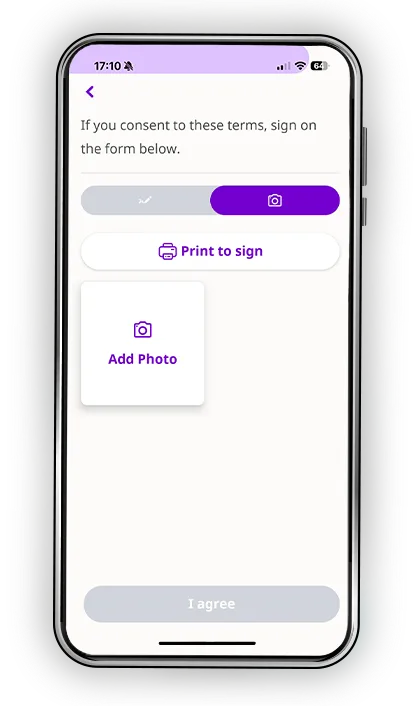
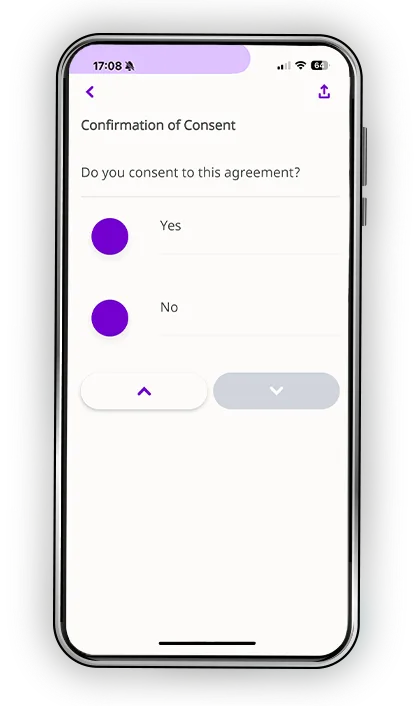
Every participant learns differently. Some process information visually. Others need time to reflect. Many simply want the reassurance of being able to revisit materials on their own schedule. Datacubed’s digital informed consent blends multimedia clarity, plain-language explanations, and structured comprehension checks to help patients truly understand the study, not just sign for it.
Features include:
- Video and graphics that simplify complex concepts
- Translations that reflect global participants and diverse literacy levels
- BYOD access so patients can review materials in the comfort of their own home
- Comprehension assessments that confirm understanding
- An interface that feels familiar, intuitive, and supportive
For Sites: A Simpler, More Confident Process
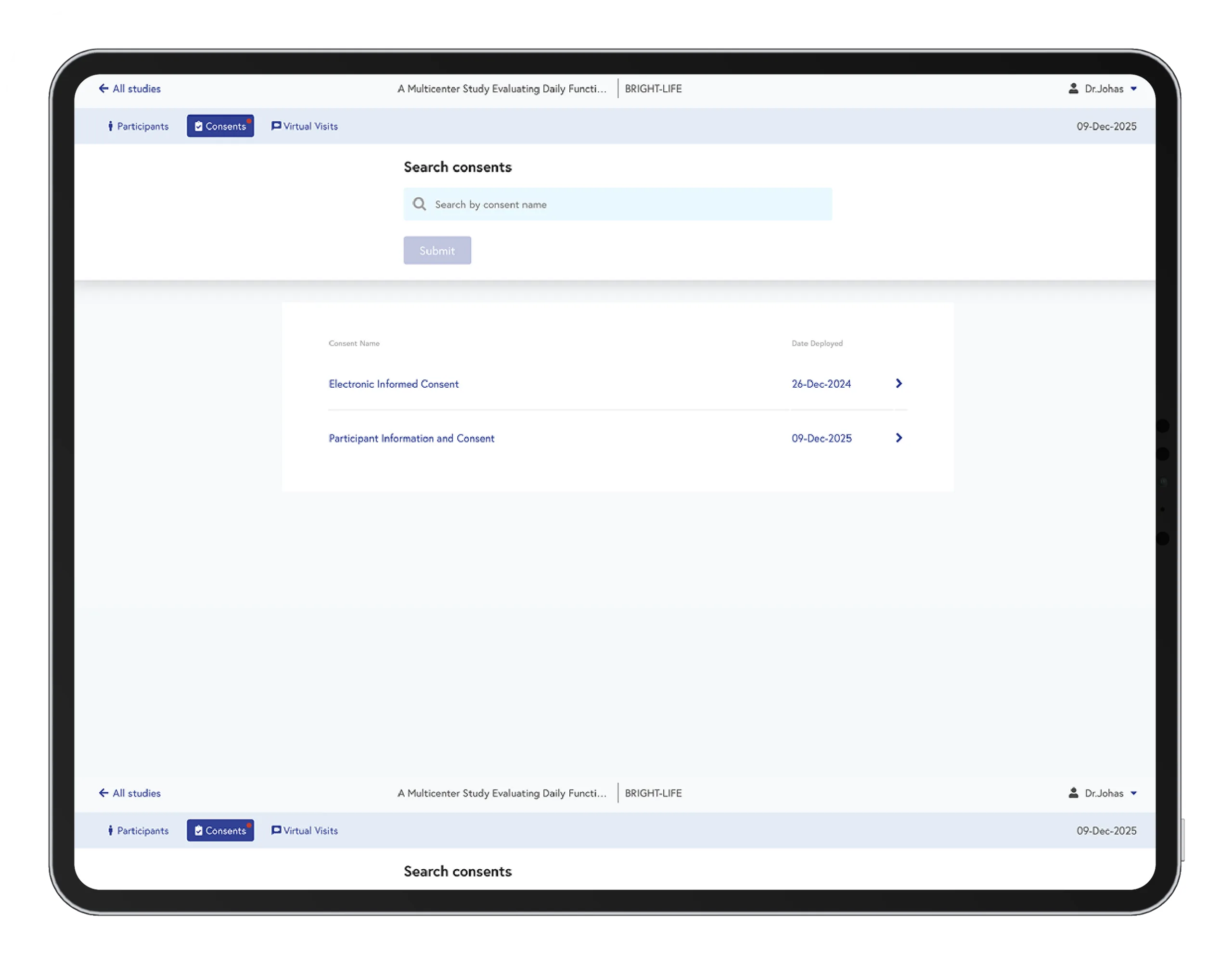
Sites often carry the heaviest burden during the consent process: collecting forms, catching discrepancies, tracking signatures, and reconciling errors. Datacubed dramatically reduces this load.
Sites benefit from:
- One place to track every participant’s consent status
- No paper. No filing cabinets. No manual reconciliation
- Clear, action-ready alerts to prevent missed signatures
- Easy access to versions, updates, and amendment tracking
- Significant reductions in time spent managing consent documents
For Sponsors & CROs: Consistency, Compliance, and Peace of Mind
Compliance is built into the core of Datacubed’s platform. Sponsors gain oversight. CROs gain efficiency. And every participant receives the same clear, consistent experience.
With Datacubed, you can rely on:
- 21 CFR Part 11, HIPAA, and GDPR compliance
- Secure, time-stamped audit trails
- Standardized workflows across every site and country
- Integrated recordkeeping that’s always inspection-ready
- Reduced risk of deviations and consent discrepancies
Build Your Trial on a Foundation of Trust
Consent sets the tone for the entire study. When participants feel supported from the start, retention rises, compliance strengthens, and teams build trust faster. Discover how transforming the consent experience doesn’t just accelerate enrollment, it builds the participant partnership that powers retention and reliable data from day one.